Electric Charge
Digital lesson: What Is Electricity?
|
Digital lesson: Electric Charge And Static Electricity?
|
|---|
Electric Charge
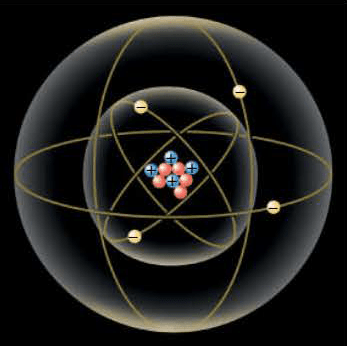
Like mass, electric charge is an intrinsic property of protons and electrons, and only two types of charge have been discovered, positive and negative. A proton has a positive charge, and an electron has a negative charge. A neutron has no net electric charge. Experiment reveals that the magnitude of the charge on the proton exactly equals the magnitude of the charge on the electron; the proton carries a charge $+e$, and the electron carries a charge $-e$. The SI unit for measuring the magnitude of an electric charge is the coulomb (C) [the definition of the coulomb depends on electric currents and magnetic fields], and $e$ has been determined experimentally to have the value $e = 1.60 \times 10^{-19}$ C.
The symbol $e$ represents only the magnitude of the charge on a proton or an electron and does not include the algebraic sign that indicates whether the charge is positive or negative.
In nature, atoms are normally found with equal numbers of protons and electrons. Usually, then, an atom carries no net charge because the algebraic sum of the positive charge of the nucleus and the negative charge of the electrons is zero. When an atom, or any object, carries no net charge, the object is said to be electrically neutral. The neutrons in the nucleus are electrically neutral particles.
Charges of larger magnitude than the charge on an electron or on a proton are built up on an object by adding or removing electrons. Thus, any charge of magnitude $q$ is an integer multiple of $e$; that is, $$q = Ne$$, where N is an integer. Because any electric charge $q$ occurs in integer multiples of elementary, indivisible charges of magnitude $e$, electric charge is said to be quantized.
How many electrons are there in one coulomb of negative charge?
Conductors and Insulators
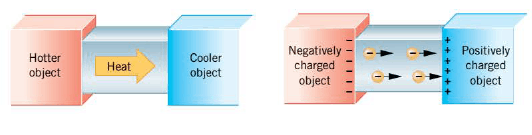 Electric charge can not only exist on an object, but it can also move through an object. However, materials differ vastly in their abilities to allow electric charge to move or be conducted through them. To help illustrate such differences in conductivity, the Figure recalls the conduction of heat through a bar of material whose ends are maintained at different temperatures. Metals conduct heat readily and, therefore, are known as thermal conductors. On the other hand, substances that conduct heat poorly are referred to as thermal insulators. A situation analogous to the conduction of heat arises when a metal bar is placed between two charged objects, as in the Figure. Electrons are conducted through the bar from the negatively charged object toward the positively charged object. Substances that readily conduct electric charge are called electrical conductors. Although there are exceptions, good thermal conductors are generally good electrical conductors. Metals such as copper, aluminum, silver, and gold are excellent electrical conductors and, therefore, are used in electrical wiring. Materials that conduct electric charge poorly are known as electrical insulators. In many cases, thermal insulators are also electrical insulators. Common electrical insulators are rubber, many plastics, and wood. Insulators, such as the rubber or plastic that coats electrical wiring, prevent electric charge from going where it is not wanted.
Electric charge can not only exist on an object, but it can also move through an object. However, materials differ vastly in their abilities to allow electric charge to move or be conducted through them. To help illustrate such differences in conductivity, the Figure recalls the conduction of heat through a bar of material whose ends are maintained at different temperatures. Metals conduct heat readily and, therefore, are known as thermal conductors. On the other hand, substances that conduct heat poorly are referred to as thermal insulators. A situation analogous to the conduction of heat arises when a metal bar is placed between two charged objects, as in the Figure. Electrons are conducted through the bar from the negatively charged object toward the positively charged object. Substances that readily conduct electric charge are called electrical conductors. Although there are exceptions, good thermal conductors are generally good electrical conductors. Metals such as copper, aluminum, silver, and gold are excellent electrical conductors and, therefore, are used in electrical wiring. Materials that conduct electric charge poorly are known as electrical insulators. In many cases, thermal insulators are also electrical insulators. Common electrical insulators are rubber, many plastics, and wood. Insulators, such as the rubber or plastic that coats electrical wiring, prevent electric charge from going where it is not wanted.
The difference between electrical conductors and insulators is related to atomic structure. As electrons orbit the nucleus, those in the outer orbits experience a weaker force of attraction to the nucleus than do those in the inner orbits. Consequently, the outermost electrons (also called the valence electrons) can be dislodged more easily than the inner ones. In a good conductor, some valence electrons become detached from a parent atom and wander more or less freely throughout the material, belonging to no one atom in particular. The exact number of electrons detached from each atom depends on the nature of the material, but is usually between one and three. When one end of a conducting bar is placed in contact with a negatively charged object and the other end in contact with a positively charged object, as in the Figure, the "free" electrons are able to move readily away from the negative end and toward the positive end. The ready movement of electrons is the hallmark of a good conductor. In an insulator the situation is different, for there are very few electrons free to move throughout the material. Virtually every electron remains bound to its parent atom. Without the "free" electrons, there is very little flow of charge when the material is placed between two oppositely charged bodies, so the material is an electrical insulator.
Digital lesson: What Are Conductors and Insulators?
|
Digital lab: How Do Conductors and Insulators Differ?
|
|---|
Charging Objects and Conservation of Electric Charge
Electricity has many useful applications that have come about because it is possible to transfer electric charge from one object to another. Usually electrons are transferred, and the body that gains electrons acquires an excess of negative charge. The body that loses electrons has an excess of positive charge. Such separation of charge occurs often when two unlike materials are rubbed together.
 For example, when an ebonite (hard, black rubber) rod is rubbed against animal fur, some of the electrons from atoms of the fur are transferred to the rod. The ebonite becomes negatively charged, and the fur becomes positively charged, as the Figure indicates.
For example, when an ebonite (hard, black rubber) rod is rubbed against animal fur, some of the electrons from atoms of the fur are transferred to the rod. The ebonite becomes negatively charged, and the fur becomes positively charged, as the Figure indicates.
Similarly, if a glass rod is rubbed with a silk cloth, some of the electrons are removed from the atoms of the glass and deposited on the silk, leaving the silk negatively charged and the glass positively charged. There are many familiar examples of charge separation, as when you walk across a nylon rug or run a comb through dry hair. In each case, objects become "electrified" as surfaces rub against one another.
When an ebonite rod is rubbed with animal fur, the rubbing process serves only to separate electrons and protons already present in the materials. No electrons or protons are created or destroyed. Whenever an electron is transferred to the rod, a proton is left behind on the fur. Since the charges on the electron and proton have identical magnitudes but opposite signs, the algebraic sum of the two charges is zero, and the transfer does not change the net charge of the fur/rod system. If each material contains an equal number of protons and electrons to begin with, the net charge of the system is zero initially and remains zero at all times during the rubbing process.
Electric charges play a role in many situations other than rubbing two surfaces together. They are involved, for instance, in chemical reactions, electric circuits, and radioactive decay. A great number of experiments have verified that in any situation, the law of conservation of electric charge is obeyed.
LAW OF CONSERVATION OF ELECTRIC CHARGE:
During any process, the net electric charge of an isolated system remains constant (is conserved).
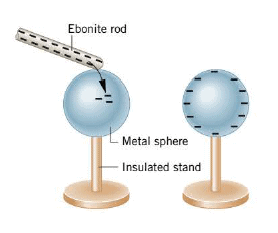 When a negatively charged ebonite rod is rubbed on a metal object, such as the sphere in the Figure, some of the excess electrons from the rod are transferred to the object. Once the electrons are on the metal sphere (where they can move readily) and the rod is removed, they repel one another and spread out over the sphere's surface. The insulated stand prevents them from flowing to the earth, where they could spread out even more. As shown in the second part of the figure, the sphere is left with a negative charge distributed over its surface. In a similar manner, the sphere would be left with a positive charge after being rubbed with a positively charged rod. In this case, electrons from the sphere would be transferred to the rod. The process of giving one object a net electric charge by placing it in contact with another object that is already charged is known as charging by contact.
When a negatively charged ebonite rod is rubbed on a metal object, such as the sphere in the Figure, some of the excess electrons from the rod are transferred to the object. Once the electrons are on the metal sphere (where they can move readily) and the rod is removed, they repel one another and spread out over the sphere's surface. The insulated stand prevents them from flowing to the earth, where they could spread out even more. As shown in the second part of the figure, the sphere is left with a negative charge distributed over its surface. In a similar manner, the sphere would be left with a positive charge after being rubbed with a positively charged rod. In this case, electrons from the sphere would be transferred to the rod. The process of giving one object a net electric charge by placing it in contact with another object that is already charged is known as charging by contact.
 [see Electric Force to understand this method to Charge Objects]
[see Electric Force to understand this method to Charge Objects]
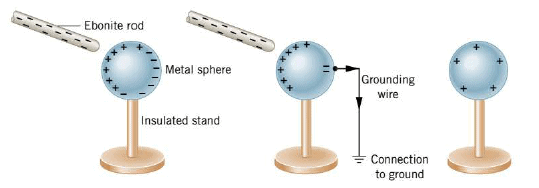
It is also possible to charge a conductor in a way that does not involve contact. In the Figure, a negatively charged rod is brought close to, but does not touch, a metal sphere. In the sphere, the free electrons closest to the rod move to the other side, as first part of the drawing indicates. As a result, the part of the sphere nearest the rod becomes positively charged and the part farthest away becomes negatively charged. These positively and negatively charged regions have been "induced" or "persuaded" to form because of the repulsive force between the negative rod and the free electrons in the sphere. If the rod were removed, the free electrons would return to their original places, and the charged regions would disappear.
Under most conditions the earth is a good electrical conductor. So when a metal wire is attached between the sphere and the ground, as in the second part of the Figure, some of the free electrons leave the sphere and distribute themselves over the much larger earth. If the grounding wire is then removed, followed by the ebonite rod, the sphere is left with a positive net charge, as the third part of the picture shows. The process of giving one object a net electric charge without touching the object to a second charged object is called charging by induction. The process could also be used to give the sphere a negative net charge, if a positively charged rod were used. Then, electrons would be drawn up from the ground through the grounding wire and onto the sphere.
If the sphere in the Figure were made from an insulating material like plastic, instead of metal, the method of producing a net charge by induction would not work, because very little charge would flow through the insulating material and down the grounding wire. However, the electric force of the charged rod would have some effect on the insulating material.
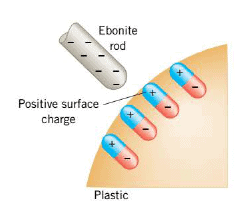 The electric force would cause the positive and negative charges in the molecules of the material to separate slightly, with the negative charges being "pushed" away from the negative rod, as the Figure illustrates. Although no net charge is created, the surface of the plastic does acquire a slight induced positive charge and is attracted to the negative rod. It is attracted in spite of the repulsive force between the negative rod and the negative charges in the plastic. This is because the negative charges in the plastic are further away from the rod than the positive charges are.
The electric force would cause the positive and negative charges in the molecules of the material to separate slightly, with the negative charges being "pushed" away from the negative rod, as the Figure illustrates. Although no net charge is created, the surface of the plastic does acquire a slight induced positive charge and is attracted to the negative rod. It is attracted in spite of the repulsive force between the negative rod and the negative charges in the plastic. This is because the negative charges in the plastic are further away from the rod than the positive charges are.
For a similar reason, one piece of cloth can stick to another in the phenomenon known as "static cling," which occurs when an article of clothing has acquired an electric charge while being tumbled about in a clothes dryer.
You don`t have permission to comment here!
Report