Heat and Heat Transfer Methods
Digital lesson: How Does Matter Change?
|
Digital Lab: How Can We Measure Temperature?
|
Digital Lab: How Does The Sun Warm Our Homes?
|
|---|
Common Temperature Scales and Thermometers
To measure temperature we use a thermometer. Many thermometers make use of the fact that materials usually expand with increasing temperature. For example, the common mercury-in-glass thermometer, which consists of a mercury-filled glass bulb connected to a capillary tube. When the mercury is heated, it expands into the capillary tube, the amount of expansion being proportional to the change in temperature. The outside of the glass is marked with an appropriate scale for reading the temperature.
Temperature Scales
A number of different temperature scales have been devised, two popular choices being the Celsius (formerly, centigrade) and Fahrenheit scales. Historically, both scales were defined by assigning two temperature points on the scale and then dividing the distance between them into a number of equally spaced intervals.
Although the Celsius and Fahrenheit scales are widely used, the Kelvin temperature scale has greater scientific significance. It was introduced by the Scottish physicist William Thompson (Lord Kelvin, 1824-1907), and in his honor each degree on the scale is called a kelvin (K).
Absolute Zero
Suppose that the absolute pressure of the gas is measured at different temperatures. If the results are plotted on a pressure-versus-temperature graph, a straight line is obtained. If the straight line is extended or extrapolated to lower and lower temperatures, the line crosses the temperature axis at -273.15 °C. In reality, no gas can be cooled to this temperature, because all gases liquify before reaching it.
In all cases, a straight line is found that extrapolates to -273.15 °C on the temperature axis, which suggests that the value of -273.15 °C has fundamental significance. The significance of this number is that it is the absolute zero point for temperature measurement. The phrase "absolute zero" means that temperatures lower than -273.15 °C cannot be reached by continually cooling a gas or any other substance. If lower temperatures could be reached, then further extrapolation of the straight line would suggest that negative absolute gas pressures could exist. Such a situation would be impossible, because a negative absolute gas pressure has no meaning. Thus, the Kelvin scale is chosen so that its zero temperature point is the lowest temperature attainable.
Thermometers
All thermometers make use of the change in some physical property with temperature. A property that changes with temperature is called a thermometric property. For example, the thermometric property of the mercury thermometer is the length of the mercury column, while in the constant-volume gas thermometer it is the pressure of the gas. Several other important thermometers and their thermometric properties can be discussed.
Interactive Demonstration: Temperature Conversion
|
Digital lesson: Temperature?
|
|---|
Thermal Expansion
Linear Thermal Expansion
Have you ever found the metal lid on a glass jar too tight to open? One solution is to run hot water over the lid, which loosens it because the metal expands more than the glass does. To varying extents, most materials expand when heated and contract when cooled. The increase in any one dimension of a solid is called linear expansion, linear in the sense that the expansion occurs along a line.
Volume Thermal Expansion
The volume of a normal material increases as the temperature increases. Most solids and liquids behave in this fashion. By analogy with linear thermal expansion, the change in volume is proportional to the change in temperature and to the initial volume, provided the change in temperature is not too large.
Digital Figure: square pattern with a hole in the center
|
|---|
Heat and Internal Energy
An object with a high temperature is said to be hot, and the word "hot" brings to mind the word "heat." Heat flows from a hotter object to a cooler object when the two are placed in contact. It is for this reason that a cup of hot coffee feels hot to the touch, while a glass of ice water feels cold. When the person touches the coffee cup, heat flows from the hotter cup into the cooler hand. When the person touches the glass, heat again flows from hot to cold, in this case from the warmer hand into the colder glass. The response of the nerves in the hand to the arrival or departure of heat prompts the brain to identify the coffee cup as being hot and the glass as being cold.
What exactly is heat? As the following definition indicates, heat is a form of energy, energy in transit from hot to cold:
Heat is energy that flows from a higher-temperature object to a lower-temperature object because of the difference in temperatures.
The heat that flows from hot to cold originates in the internal energy of the hot substance. The internal energy of a substance is the sum of the molecular kinetic energy (due to random motion of the molecules), the molecular potential energy (due to forces that act between the atoms of a molecule and between molecules), and other kinds of molecular energy. When heat flows in circumstances where no work is done, the internal energy of the hot substance decreases and the internal energy of the cold substance increases. Although heat may originate in the internal energy supply of a substance, it is not correct to say that a substance contains heat. The substance has internal energy, not heat. The word "heat" only refers to the energy actually in transit from hot to cold.
The next sections consider some effects of heat. For instance, when preparing spaghetti, the first thing that a cook does is to heat the water. Heat from the stove causes the internal energy of the water to increase. Associated with this increase is a rise in temperature. After a while, the temperature reaches 100 °C, and the water begins to boil. During boiling, the added heat causes the water to change from a liquid to a vapor phase (steam). The next section investigates how the addition (or removal) of heat causes the temperature of a substance to change. Then, discusses the relationship between heat and phase change, such as that which occurs when water boils.
Heat with Temperature Change: Specific Heat Capacity
Greater amounts of heat are needed to raise the temperature of solids or liquids to higher values. A greater amount of heat is also required to raise the temperature of a greater mass of material. Similar comments apply when the temperature is lowered, except that heat must be removed. For limited temperature ranges, experiment shows that the heat $Q$ is directly proportional to the change in temperature $\Delta T$ and to the mass $m$. These two proportionalities are expressed in Equation, with the help of a proportionality constant $c$ that is referred to as the specific beat capacity of the material.
The principle of conservation of energy states that energy can be neither created nor destroyed, but can only be converted from one form to another. We dealt with kinetic and potential energies before. In here we have expanded our concept of energy to include heat, which is energy that flows from a higher-temperature object to a lower temperature object because of the difference in temperature. No matter what its form, whether kinetic energy, potential energy, or heat, energy can be neither created nor destroyed. This fact governs the way objects at different temperatures come to an equilibrium temperature when they are placed in contact. If there is no heat loss to the external surroundings, the amount of heat gained by the cooler objects equals the amount of heat lost by the hotter ones, a process that is consistent with the conservation of energy. Just this kind of process occurs within a thermos. A perfect thermos would prevent any heat from leaking out or in. However, energy in the form of heat can flow between materials inside the thermos to the extent that they have different temperatures; for example, between ice cubes and warm tea. The transfer of energy continues until a common temperature is reached at thermal equilibrium.
The kind of heat transfer that occurs within a thermos of iced tea also occurs within a calorimeter, which is the experimental apparatus used in a technique known as calorimetry. a calorimeter is essentially an insulated container. It can be used to determine the specific heat capacity of a substance.
Heat with Phase Changes: Latent Heat
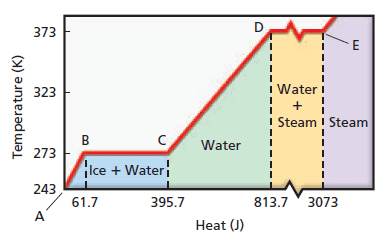
Surprisingly, there are situations in which the addition or removal of heat does not cause a temperature change. Consider a well-stirred glass of iced tea that has come to thermal equilibrium. Even though heat enters the glass from the warmer room, the temperature of the tea does not rise above 0°C as long as ice cubes are present. Apparently the heat is being used for some purpose other than raising the temperature. In fact, the heat is being used to melt the ice, and only when all of it is melted will the temperature of the liquid begin to rise.
Equilibrium Between Phases of Matter
Under specific conditions of temperature and pressure, a substance can exist at equilibrium in more than one phase at the same time. The pressure of the vapor that coexists in equilibrium with the liquid is called the equilibrium vapor pressure of the liquid.
Humidity
Air is a mixture of gases, including nitrogen, oxygen, and water vapor. The total pressure of the mixture is the sum of the partial pressures of the component gases. The partial pressure of a gas is the pressure it would exert if it alone occupied the entire volume at the same temperature as the mixture. The partial pressure of water vapor in air depends on weather conditions . It can be as low as zero or as high as the equilibrium vapor pressure of water at the given temperature.
To provide an indication of how much water vapor is in the air, weather forecasters usually give the relative humidity. If the relative humidity is too low, the air contains such a small amount of water vapor that skin and mucous membranes tend to dry out. If the relative humidity is too high, especially on a hot day, we become very uncomfortable and our skin feels "sticky."
Animated Physics: Heat
|
Digital Figure: Equilibrium
|
Digital Investigations: Phase Changes Physics
|
|---|---|---|
Animated Physics: Specific Heat and Latent Heat
|
Interactive Demonstration: Calorimetry
|
Solution Tutor: Calorimetry
|
Digital Lab: How Can The State Of Matter Change?
|
Digital lesson: What Are Solids, Liquids, And Gases?
|
Digital Lab: How Does Water Change?
|
Digital lesson: Changes Of State
|
Digital Lab: How Are Temperature And Kinetic Energy Related?
|
Digital Lab: Temperature And Thermal Energy
|
The Transfer of Heat
When heat is transferred to or from a substance, the internal energy of the substance can change, as we saw. This change in internal energy is accompanied by a change in temperature or a change in phase. The transfer of heat affects us in many ways. For instance, within our homes furnaces distribute heat on cold days, and air conditioners remove it on hot days. Our bodies constantly transfer heat in one direction or another, to prevent the adverse effects of hypo- and hypertherrnia. And virtually all our energy originates in the sun and is transferred to us over a distance of 150 million kilometers through the void of space. Today's sunlight provides the energy to drive photosynthesis in the plants that provide our food and, hence, metabolic energy. Ancient sunlight nurtured the organic matter that became the fossil fuels of oil, natural gas, and coal. Here we examines the three processes by which heat is transferred: convection, conduction, and radiation.
Convection
When part of a fluid is warmed, such as the air above a fire, the volume of that part of the fluid expands, and the density decreases. According to Archimedes' principle, the surrounding cooler and denser fluid exerts a buoyant force on the warmer fluid and pushes it upward. As warmer fluid rises, the surrounding cooler fluid replaces it. This cooler fluid, in turn, is warmed and pushed upward. Thus, a continuous flow is established, which carries along heat. Whenever heat is transferred by the bulk movement of a gas or a liquid, the heat is said to be transferred by convection. The fluid flow itself is called a convection current.
Conduction
Anyone who has fried a hamburger in an all-metal skillet knows that the metal handle becomes hot. Somehow, heat is transferred from the burner to the handle. Clearly, heat is not being transferred by the bulk movement of the metal or the surrounding air, so convection can be ruled out. Instead, heat is transferred directly through the metal by a process called conduction.
Radiation
Energy from the sun is brought to earth by large amounts of visible light waves, as well as by substantial amounts of infrared and ultraviolet waves. These waves are known as electromagnetic waves, a class that also includes the microwaves used for cooking and the radio waves used for AM and FM broadcasts. The sunbather feels hot because her body absorbs energy from the sun's electromagnetic waves. Anyone who has stood by a roaring fire or put a hand near an incandescent light bulb has experienced a similar effect. Thus, fires and light bulbs also emit electromagnetic waves, and when the energy of such waves is absorbed, it can have the same effect as heat.
The process of transferring energy via electromagnetic waves is called radiation, and, unlike convection or conduction, it does not require a material medium. Electromagnetic waves from the sun, for example, travel through the void of space during their journey to earth.
Digital simulations: Heat conduction
|
Digital Figure: Heat is conducted through the bar
|
Digital Figure: radiant energy
|
|---|---|---|
Digital lesson: How Does Heat Move?
|
Digital Figure: thermos bottle
|
Concept Map: Heat
|
Digital Lab: How Is Heat Produced?
|
Digital lesson: Thermal Energy And Heat
|
You don`t have permission to comment here!
Report