Kinetic Theory of Gases
Gases are easily compressed. You will note that gases have the largest coefficients of volume expansion. The large coefficients mean that gases expand and contract very rapidly with temperature changes. In addition, you will note that most gases expand at the same rate. This raises the question as to why gases should all act in nearly the same way, when liquids and solids have widely varying expansion rates. The answer lies in the large separation of atoms and molecules in gases, compared to their sizes. Because atoms and molecules have large separations, forces between them can be ignored, except when they collide with each other during collisions. The motion of atoms and molecules (at temperatures well above the boiling temperature) is fast, such that the gas occupies all of the accessible volume and the expansion of gases is rapid. In contrast, in liquids and solids, atoms and molecules are closer together and are quite sensitive to the forces between them.
At room temperatures, collisions between atoms and molecules can be ignored. In this case, the gas is called an ideal gas, in which case the relationship between the pressure, volume, and temperature is given by the equation of state called the ideal gas law.
We have developed macroscopic definitions of pressure and temperature. Pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion.
The Amount of a Substance in Moles
Often, we wish to compare the mass of one atom with another. To facilitate the comparison, a mass scale known as the atomic mass scale has been established. To set up this scale, a reference value (along with a unit) is chosen for one of the elements. The unit is called the atomic mass unit (symbol: u). By international agreement, the reference element is chosen to be the most abundant type or isotope of carbon, which is called carbon-12. Its atomic mass is defined to be exactly twelve atomic mass units, or 12 u. The molecular mass of a molecule is the sum of the atomic masses of its atoms.
Macroscopic amounts of materials contain large numbers of atoms or molecules. Even in a small volume of gas, 1 cm3 , for example, the number is enormous. It is convenient to express such large numbers in terms of a single unit, the gram-mole, or simply the mole (symbol: mol). One gram-mole of a substance contains as many particles (atoms or molecules) as there are atoms in 12 grams of the isotope carbon-12. Experiment shows that 12 grams of carbon-12 contain $6.022 \times 10^{23}$ atoms. The number of atoms per mole is known as Avogadro's number NA, after the Italian scientist Amedeo Avogadro (1776- 1856). Although defined in terms of carbon atoms, the concept of a mole can be applied to any collection of objects by noting that one mole contains Avogadro's number of objects. The mole is the SI base unit for expressing "the amount of a substance."
Digital Figure: periodic table
|
|---|
The Ideal Gas Law
An ideal gas is an idealized model for real gases that have sufficiently low densities. The condition of low density means that the molecules of the gas are so far apart that they do not interact (except during collisions that are effectively elastic). The ideal gas law expresses the relationship between the absolute pressure, the Kelvin temperature, the volume, and the number of moles of the gas.
Interactive Demonstration: Work Done on or by a Gas
|
|---|
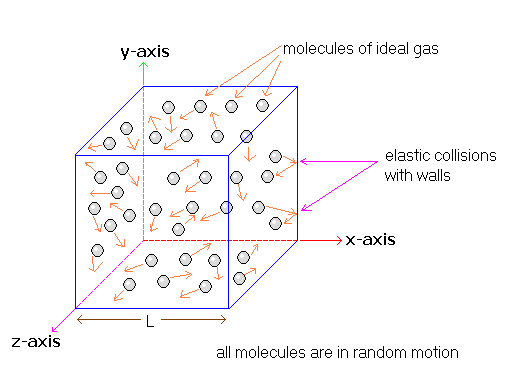
Kinetic Theory of Gases
As useful as it is, the ideal gas law provides no insight as to how pressure and temperature are related to properties of the molecules themselves, such as their masses and speeds. To show how such microscopic properties are related to the pressure and temperature of an ideal gas, this section examines the dynamics of molecular motion. The pressure that a gas exerts on the walls of a container is due to the force exerted by the gas molecules when they collide with the walls. Therefore, we will begin by combining the notion of collisional forces exerted by a fluid with Newton's second and third laws of motion. These concepts will allow us to obtain an expression for the pressure in terms of microscopic properties. We will then combine this with the ideal gas law to show that the average translational kinetic energy of a particle in an ideal gas is $\bar{KE} = \frac{3}{2} kT$, where k is Boltzmann's constant and T is the Kelvin temperature. In the process, we will also see that the internal energy of a monatomic ideal gas is $U = \frac{3}{2} nRT$, where n is the number of moles and R is the universal gas constant.
Digital Investigations: Kinetic Theory
|
|---|
Diffusion
You can smell the fragrance of a perfume at some distance from an open bottle because perfume molecules leave the space above the liquid in the bottle, where they are relatively concentrated, and spread out into the air, where they are less concentrated. During their journey, they collide with other molecules, so their paths resemble the zigzag paths characteristic of Brownian motion. The process in which molecules move from a region of higher concentration to one of lower concentration is called diffusion. Diffusion also occurs in liquids and solids, for example ink diffusing through water. However, compared to the rate of diffusion in gases, the rate iis generally smaller in liquids and even smaller in solids. The host medium, such as the air or water in the examples above, is referred to as the solvent, while the diffusing substance, like the perfume molecules or the ink, is known as the solute. Relatively speaking, diffusion is a slow process, even in a gas.
Digital Figure: Using diffusion
|
|---|
You don`t have permission to comment here!
Report