Carbon monoxide gas with a molecular mass of 28.0 kg/kmol and an initial temperature of 200 K is confined to the left side of a sealed container. Diatomic nitrogen gas with a molecular mass of 28.0 kg/kmol and an initial temperature of 400 K is confined to the right side of the sealed container, as shown in the figure.
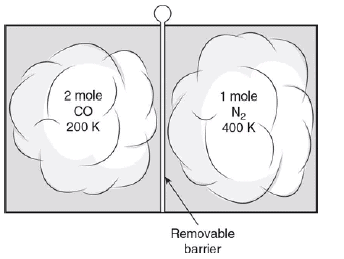
Separating the gases is a removable barrier. When the barrier is removed, the two gases mix.
(A) Describe the temperature changes in the two gases.
(B) Describe any movement of thermal energy over a long period of time. Explain the microscopic process that determines this process.
(C) Sketch the initial molecular speed distributions of the two gases on the axis. Label each gas. Explain any differences in the speed distribution of the two gases.
(D) Sketch the final molecular speed distributions of the two gases on the axis. Label each gas. Explain the changes in the graph from the initial condition. Also explain any differences in the speed distributions of the two gases.
You don`t have permission to comment here!
Report