from a homework
Degree Level: Pre-College
Question:
Use the figure to answer the following questions.
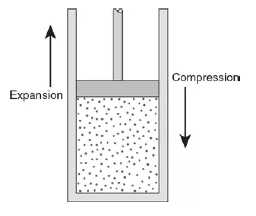
(A) Explain why work done on the gas means the gas is being compressed and why work done by the gas means the gas is expanding.
(B) Explain why doing work on the gas by pushing the piston to compress the gas increases the molecular kinetic energy of the gas.
(C) Use the behavior of atoms to explain why a gas will lose internal thermal energy when the gas expands by moving a piston.
(D) How do we calculate the work done when a gas expands or is compressed?
You don`t have permission to comment here!
Report