Laws of Thermodynamic
In thermodynamics the collection of objects on which attention is being focused is called the system, while everything else in the environment is called the surroundings. For example, the system in an automobile engine could be the burning gasoline, whereas the surroundings would then include the pistons, the exhaust system, the radiator, and the outside air. The system and its surroundings are separated by walls of some kind. Walls that permit heat to flow through them, such as those of the engine block, are called diathermal walls. Perfectly insulating walls that do not permit heat to flow between the system and its surroundings are known as adiabatic walls.
To understand what the laws of thermodynamics have to say about the relationship between heat and work, it is necessary to describe the physical condition or state of a system. We might be interested, for instance, in the hot air within one of the balloons. The hot air itself would be the system, and the skin of the balloon provides the walls that separate this system from the surrounding cooler air. The state of the system would be specified by giving values for the pressure, volume, temperature, and mass of the hot air.
There are four laws of thermodynamics. We begin with the one known as the zeroth law and then consider the remaining three.
The Zeroth Law of Thermodynamics
The zeroth law of thermodynamics deals with the concept of thermal equilibrium. Two systems are said to be in thermal equilibrium if there is no net flow of heat between them when they are brought into thermal contact. temperature is the indicator of thermal equilibrium in the sense that there is no net flow of heat between two systems in thermal contact that have the same temperature.
THE ZEROTH LAW OF THERMODYNAMICS: Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with each other.
The First Law of Thermodynamics
The atoms and molecules of a substance have kinetic and potential energy. These and other kinds of molecular energy constitute the internal energy of a substance. When a substance participates in a process involving energy in the form of work and heat, the internal energy of the substance can change. The relationship between work, heat, and changes in the internal energy is known as the first law of thermodynamics. We will now see that the first law of thermodynamics is an expression of the conservation of energy.
THE FIRST LAW OF THERMODYNAMICS: The internal energy of a system changes from an initial value $U_i$ to a final value of $U_f$ due to heat $Q$ and work $W$:
$$\Delta U = U_f - U_f = Q - W$$
$Q$ is positive when the system gains heat and negative when it loses heat. $W$ is positive when work is done by the system and negative when work is done on the system.
Thermal Processes
A system can interact with its surroundings in many ways, and the heat and work that come into play always obey the first law of thermodynamics. We will introduces four common thermal processes. In each case, the process is assumed to be quasi-static, which means that it occurs slowly enough that a uniform pressure and temperature exist throughout all regions of the system at all times.
An isobaric process is one that occurs at constant pressure.
Another common thermal process is an isochoric process, one that occurs at constant volume.
A third important thermal process is an isothermal process, one that takes place at constant temperature.
Last, there is the adiabatic process, one that occurs without the transfer of heat.
Thermal Processes Using an Ideal Gas
Isothermal expansion or compression of an ideal gas:
$$ W = n R T \ln \left( \dfrac{V_f}{V_i} \right)$$
Adiabatic expansion or compression of a monatomic ideal gas:
$$ W = \frac{3}{2} n R \left( T_i - T_f \right)$$
Specific Heat Capacities
The first law of thermodynamics is used to gain an understanding of the factors that determine the specific heat capacity of a material.
Animated Physics: First Law of Thermodynamics
|
Digital Figure: isobaric process
|
Digital Figure: system gains energy
|
|---|---|---|
Interactive Demonstration: The First Law of Thermodynamics
|
The Second Law of Thermodynamics
Ice cream melts when left out on a warm day. A cold can of soda warms up on a hot day at a picnic. Ice cream and soda never become colder when left in a hot environment, for heat always flows spontaneously from hot to cold, and never from cold to hot. The spontaneous flow of heat is the focus of one of the most profound laws in all of science, the second law of thermodynamics.
THE SECOND LAW OF THERMODYNAMICS: Heat flows spontaneously from a substance at a higher temperature to a substance at a lower temperature and does not flow spontaneously in the reverse direction.
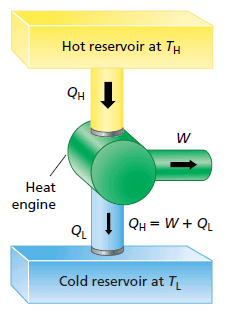
Heat Engines
A heat engine is any device that uses heat to perform work. It has three essential features:
1. Heat is supplied to the engine at a relatively high input temperature from a place called the hot reservoir.
2. Part of the input heat is used to perform work by the working substance of the engine, which is the material within the engine that actually does the work (e.g., the gasoline-air mixture in an automobile engine).
3. The remainder of the input heat is rejected to a place called the cold reservoir, which has a temperature lower than the input temperature.
Carnot's Principle and the Carnot Engine
What is it that allows a heat engine to operate with maximum efficiency? The French engineer Sadi Carnot (1796-1832) proposed that a heat engine has maximum efficiency when the processes within the engine are reversible. A reversible process is one in which both the system and its environment can be returned to exactly the states they were in before the process occurred.
Today, the idea that the efficiency of a heat engine is a maximum when the engine operates reversibly is referred to as Carnot's principle.
Refrigerators, Air Conditioners, and Heat Pumps
The natural tendency of heat is to flow from hot to cold, as indicated by the second law of thermodynamics. However, if work is used, heat can be made to flow from cold to hot, against it's natural tendency. Refrigerators, air conditioners, and heat pumps are, in fact, devices that do just that. These devices use work to extract heat from the cold reservoir and deposit heat into the hot reservoir. Generally speaking, such a process is called a refrigeration process.
Entropy
THE SECOND LAW OF THERMODYNAMICS STATED IN TERMS OF ENTROPY:
The total entropy of the universe does not change when a reversible process occurs and increases when an irreversible process occur.
Digital simulations: The Efficiencey of a Carnot Engine
|
Digital Figure: heat engine
|
Interactive Demonstration: Heat-Engine Efficiency
|
|---|
The Third Law of Thermodynamics
The third law of thermodynamics indicates that it is impossible to reach a temperature of absolute zero.
THE THIRD LAW OF THERMODYNAMICS: It is not possible to lower the temperature of any system to absolute zero in a finite number of steps.
Concept Map: Thermodynamics
|
|---|
You don`t have permission to comment here!
Report