A mole of ideal gas is enclosed in a cylinder with a movable piston with a cross-sectional area of 1 × 10–2 m2. The gas is taken through a thermodynamic process, as shown in the figure.
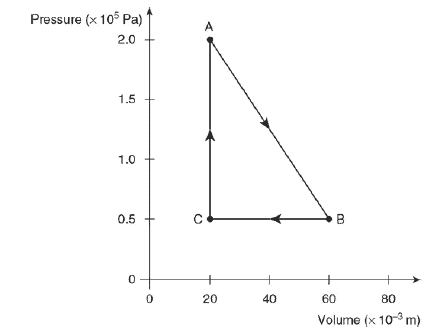
(a) Calculate the temperature of the gas at state A, and describe the microscopic property of the gas that is related to the temperature.
(b) Calculate the force of the gas on the piston at state A, and explain how the atoms of the gas exert this force on the piston.
(c) Predict qualitatively the change in the internal energy of the gas as it is taken from state B to state C. Justify your prediction.
(d) Is heat transferred to or from the gas as it is taken from state B to state C? Justify your answer.
(e) Discuss any entropy changes in the gas as it is taken from state B to state C. Justify your answer.
(f) Calculate the change in the total kinetic energy of the gas atoms as the gas is taken from state C to state A.
(g) On the axis provided, sketch and label the distribution of the speeds of the atoms in the gas for states A and B.
You don`t have permission to comment here!
Report