Two moles of a gas is taken through the thermodynamic process ABCA, as shown in the figure.
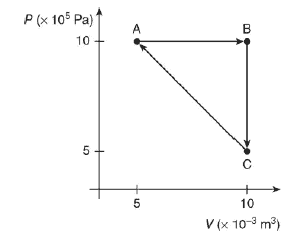
(A) Rank the work in the steps of the process from most positive to most negative.
(B) Rank the change in temperature of the gas for each step in the process from most positive to most negative.
(C) Rank the thermal energies of the points A, B, and C from greatest to least.
(D) Calculate the temperature of point B.
(E) Calculate the change in temperature for the entire cycle ABCA.
(F) Calculate the work done in process C?A.
(G) Calculate the heat flow for process C?A.
You don`t have permission to comment here!
Report